|
CE Certification and Testing Solutions for Eyewear Protection and Face Shield during the COVID-19 Period
In the context of the current COVID-19 global outbreak as well as the rapid spread of the virus across various regions of the EU and American counties, the demand for personal protective equipment, such as protective surgical masks, gloves, eyewear protection and face shield, has seen an exponential growth.
China is a major country of producing and exporting key anti-pandemic supplies. According to the recommendations from Commission Recommendation (EU)2020/403 of 13 March 2020, the export of eyewear protection and face shield (including those with the function of virus prevention and protection) shall comply with PPE Regulation (EU)2016/425. At present, can non-CE marketing product be exported to the EU? How should we interpret the recommendations of the European Commission? We have sorted out the following key points for you.
-
Notified bodies should thus assess whether products manufactured in line with other technical solutions, such as the ones contained in the WHO recommendations on the appropriate selection of PPE also meet the applicable essential health and safety requirements. When delivering the conformity assessment certificates, notified bodies are required to inform their notifying authorities and may also be required to inform other notified bodies of the certificates they have issued.
Expert’s Opinion: Export of PPE shall still satisfy the corresponding testing requirements of CE certification, undergo testing and be certified by notified bodies.
-
Accordingly, to address the shortage of PPE necessary in the context of the COVID-19 outbreak, where non-CE marked PPE are intended to enter the EU market, the relevant market surveillance authorities should evaluate the products and, if they are found to be compliant with the essential health and safety requirements laid down by the relevant Regulation should take measures allowing the placing of such PPE on the Union market for a limited period of time or while the conformity assessment procedure with the notified body is being carried out.
Expert’s Opinion: Export of PPE still requires CE certification, with delay in the procedures accepted by relevant authorities only in some circumstances.
-
Considering that certain types of PPE or medical devices that are used in the context of the COVID-19 outbreak, may also be used for other purposes, it is necessary that Member States take all appropriate measures to ensure that PPE or medical devices not bearing the CE marking, which may be placed on the Union market in accordance are only made available to healthcare workers.
Expert’s Opinion: Authorities have the right to purchase PPE temporarily without CE marking to be used by medical workers (as mentioned in Point 2 above), but suppliers shall not be exempted from CE marking for such products. Circulation of such products without CE marking in the market will be subject to such control actions as notification, recall or punishment.
For health management and service personnel in public places, such as medical workers, cleaners, anti-epidemic management personnel and management and service personnel of crowded places, eyewear protection and face shield are important devices to protect health and safety, in addition to surgical masks, which effectively reduce the risk of virus infection. Eyewear protection and face shield products entering global markets are subject to the following standards according to different uses.
Standers of Eyewear Protection and Face Shield:
|
Export Destination
|
Regulation/standard
|
Certification
|
|
EU
|
2016/425,
EN 166 Personal eye-protection —Specifications
|
CE
|
|
U.S.
|
FDA 21 cfr 801.410,
29 CFR § 1910.133,
ANSI Z87.1 American National Standard Occupational and Educational Personal Eye and Face Protection
|
FDA
|
|
Australia
|
AS/NZS 1337.1 Personal Eye Protection Eye and Face Protectors for Occupational Applications
|
SAI
|
|
Canada
|
CSA Z94.3 Eye and Face Protectors
|
—
|
|
China
|
GB 14866 The Specifications for Personal Eye-protectors
|
—
|
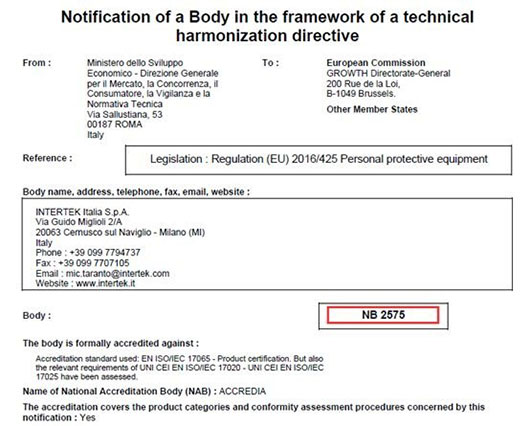
Intertek is a leading Total Quality Assurance provider to industries worldwide. Whether in the past or the current pandemic period, Intertek has rendered a suite of professional, precise, fast and enthusiastic testing, certification and consultation services to a number of PPE manufacturers and traders. By giving more effective guarantee for the quality of PPE products, it has earned trust from international renowned buyers. On top of that, Intertek owns NB-2575 in Italy and NB-0362 in UK, two notified bodies for PPE CE certification that provide enterprises with proactive CE certification solutions for PPE products.

For More Information on PPE, Please Contact Us Now:
SC
0755-2602 0151
0755-2602 0369
020-3210 2492
EC
0577-8555 6051
021-5339 6228
NC
022-8371 4973
Email:
service.china@intertek.com

|